The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Study and Its Implications for Patients With Chronic Schizophrenia

- Jonathan Hickman, PMHNP
What do these results mean for a practicing PMHNP?
"Schizophrenia can be a very difficult diagnosis to treat. The CATIE trials provide useful evidence-based data that can guide clinicians in the selection of further pharmacological interventions for patients that are not fully responding to current treatment or experiencing side effects."
NP Psych Navigator contributors are paid consultants of AbbVie Inc.
Why was the research needed?
Schizophrenia is a challenging psychiatric disorder that is treated using different types of antipsychotic medications. Patients often try older antipsychotic medications (ie, first-generation antipsychotics [FGAs], or typical antipsychotics) and newer medications (ie, second-generation antipsychotics [SGAs], or atypical antipsychotics). However, before the CATIE study, the comparative efficacy and side-effect profiles of these medications were unclear.1,2
While both FGAs and SGAs had been evaluated in clinical trials that demonstrated their efficacy and safety, these trials were short and did not include real-world populations. The trials also did not assess how patients would respond to a wide range of different antipsychotic medications.2
As treatment switches are often made based on patient preference and side effects, research comparing the safety and efficacy of antipsychotic medications in the long term among a diverse population was needed to inform clinical decisions for patients who did not respond to previous treatment.1
What did the researchers do?
The CATIE study, which was funded by the National Institute of Mental Health, was designed to evaluate the effectiveness of conventional (FGAs) and atypical (SGAs) antipsychotics in patients with schizophrenia.1 Adults 18 to 65 years old with schizophrenia who were eligible for oral antipsychotic treatment were enrolled. The study was conducted between January 2001 and December 2004 across 57 clinical sites in the United States.1
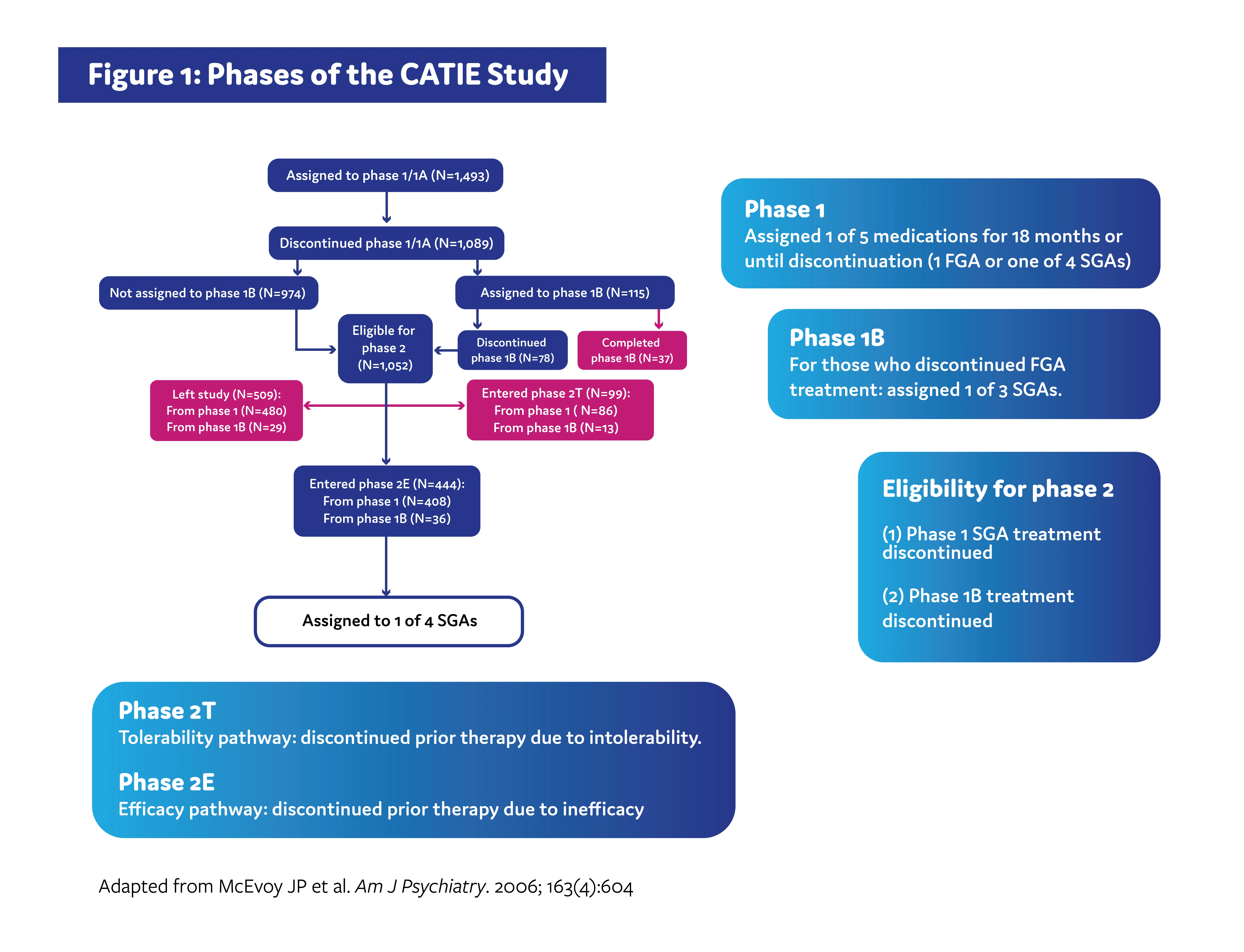
The CATIE study was carried out across multiple phases (Figure 1). In phase 1, patients were randomized to receive either an SGA or an FGA. Patients remained on treatment for 18 months or until discontinuation for any reason. Patients who discontinued treatment in phase 1 due to inadequate symptom control were recommended to enroll in the phase 2 efficacy pathway, and patients who discontinued due to intolerable side effects were recommended to enroll in the phase 2 tolerability pathway. However, patients were ultimately able to choose which pathway to take.1,3
The efficacy pathway was designed to determine if an older SGA would be more effective than SGAs that were newer in comparison, while the tolerability study was designed to determine if any of the 4 SGAs assigned during phase 1 would be beneficial in patients who previously experienced intolerability.3
The primary outcome of both pathways was time until treatment discontinuation for any reason.1,4
What were the key results of the study?
Patients in the IMDCP who met the inclusion criteria for this analysis had an average age of 40.3 years, and 63.4% were female. Of the 263 participants who reported their race, 87.1% were Caucasian, 4.6% were Asian, 4.2% were Black/African American, and 4.2% were other races. Of the 280 who reported employment status, 45.7% were employed, 35.7% were unemployed or disabled, 11.8% were students, and 6.8% were retired or homemakers.1
The study authors found that self-rated cognitive impairment significantly correlated with anhedonia (r = 0.131, p = 0.012). Cognitive dysfunction also correlated with total depression symptom severity as assessed by total MADRS score (r = 0.147, p = 0.005). Anhedonia and self-rated cognitive dysfunction remained significantly correlated after adjusting for illness severity (ie, CGI-S; r = 0.162, p = 0.007).1
The data from this study indicated that individuals with anhedonia were more likely to report deficits in measures of cognitive function. This relationship is dissociable from the overall severity of MDD since significant correlation remained even after adjusting for illness severity.1
Limitations
Study limitations included1:
- Factors relevant to large, observational, cross-sectional cohorts (eg, absence of control for comorbidities, illness course, treatment assignments)
- Missing data from participants
- A lack of longitudinal follow-up
- Utilization of the MADRS as a proxy for anhedonia in place of an independently validated measure
- Self-reported cognitive measure in place of a comprehensive neuropsychological function assessment; however, the researchers note that the ASRS does subjectively evaluate cognitive function domains that are frequently reported as abnormal in MDD
- Lack of a control group
- Cohort of participants (ie, individuals using services at tertiary-based mood disorder centers) may limit generalizability; however, the researchers note that the subjects represent real patients seeking treatment for MDD since they were not selected based on any additional criteria
Why are these results important?
The results of this study provide preliminary clinical data supporting the hypothesis that anhedonia may correlate with cognitive dysfunction in MDD, independent of illness severity. This correlation was significant yet modest, indicating that they may be separate phenomena with genesis from common neurobiological systems. The potential relationship between these symptoms may help clinicians identify MDD patients who are at higher risk of cognitive decline over time.1
Across neuropsychological conditions, anhedonia is a challenging symptom that can be associated with poorer treatment response, illness chronicity, and increased suicide risk.5 Additionally, issues with cognition have been shown to play a major role in mediating the impact on functional impairment in MDD and other disorders.6
Focusing on both anhedonia and cognitive dysfunction may be an important MDD management goal that can help patients achieve better psychosocial outcomes, workplace functioning, and other patient-reported outcomes such as quality of life.7,8
What’s next?
Further studies using validated instruments are needed to better assess the overlap of anhedonia and cognitive function in patients with MDD. Additionally, examining the subdomains of anhedonia (eg, desire, anticipation, motivation, effort, pleasure, learning of stimulus-reward associations) could provide deeper insights.1,9
References
- Stroup TS, Lieberman JA, McEvoy JP, et al. Am J Psychiatry. 2006;163(4):611- 622.
- National Institute of Mental Health. Questions and answers about the NIMH Clinical Antipsychotic Trials of Intervention Effectiveness Study (CATIE) — phase 1 results. 2005.
- National Institute of Mental Health. Questions and Answers About the NIMH Clinical Antipsychotic Trials of Intervention Effectiveness Study (CATIE) — Phase 2 Results. 2006.
- McEvoy JP, Lieberman JA, Stroup TS, et al. Am J Psychiatry. 2006;163(4):600- 610.
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-23.
This summary was prepared independently of the study’s authors.
This resource is intended for educational purposes only and is intended for US healthcare professionals. Healthcare professionals should use independent medical judgment. All decisions regarding patient care must be handled by a healthcare professional and be made based on the unique needs of each patient.
ABBV-US-00739-MC, Version 2.0
Approved 01/2024
AbbVie Medical Affairs
